Okami Medical Announces FDA 510(k) Clearance of the LOBO-7 and LOBO-9 Vascular Occluders To Address A Wide Range Of Peripheral Embolization Cases
Aliso Viejo, CA – June 7, 2022 – Okami Medical Inc., a medical device company focused on developing innovative solutions that address key unmet clinical needs in peripheral vascular intervention, today announced U.S. Food and Drug Administration (FDA) 510(k) clearance of the LOBO-7 and LOBO-9 Vascular Occluders, the latest addition to the company’s LOBO® Vascular Occlusion System.
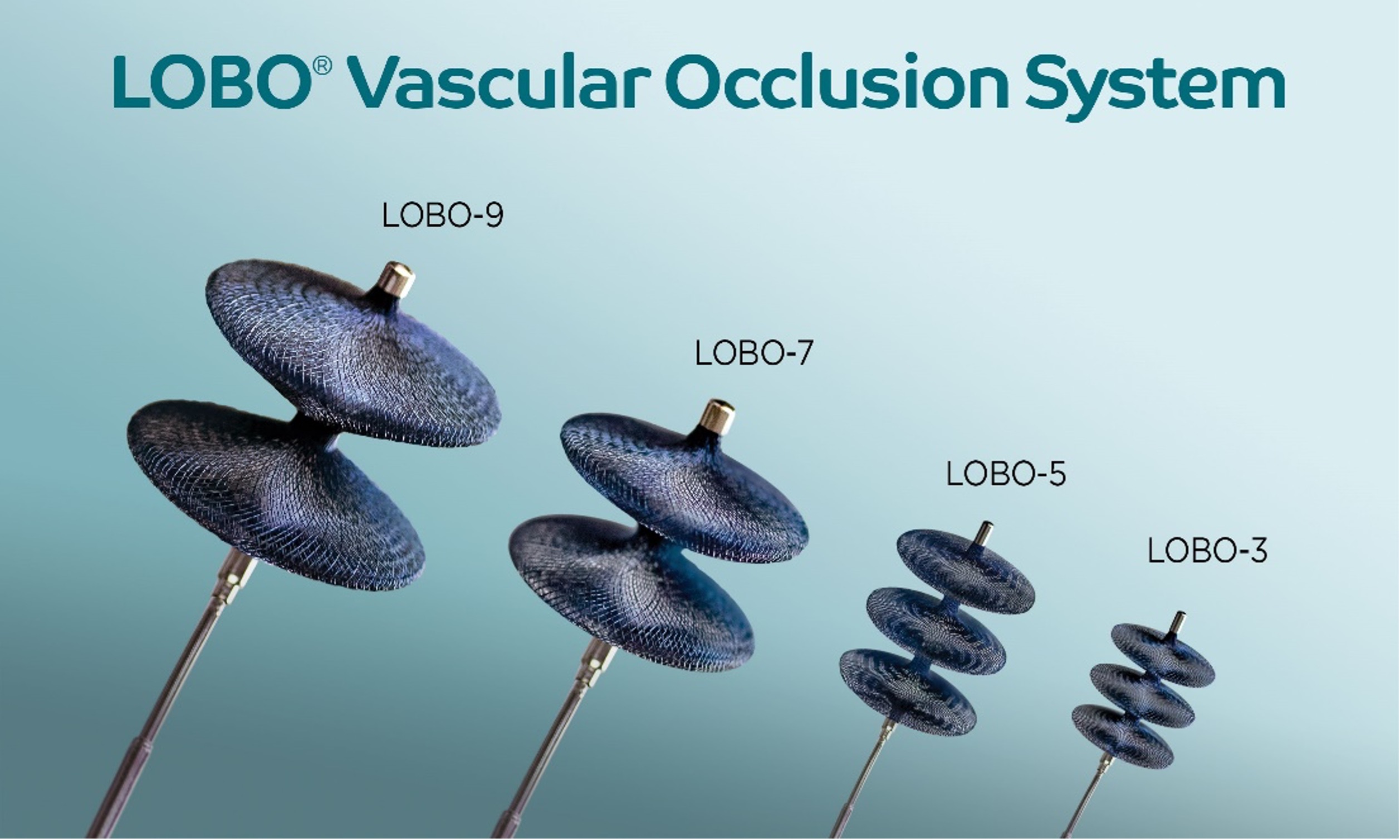
The LOBO (LOw-profile Braided Occluder) system, purpose-built for fast and complete occlusion of a diverse set of peripheral arterial targets, now includes LOBO-3, LOBO-5, LOBO-7 and LOBO-9. The LOBO occluders integrate patented HDBRAID® technology and an innovative design to provide interventional physicians with a One-and-Done solution for embolization. The highly occlusive braided structure rapidly reduces flow and enables single-device occlusions of blood vessels throughout the body. LOBO-7 and LOBO-9 are intended for use in 5 to 7 mm and 7 to 9 mm diameter vessels, respectively. LOBO-3 and LOBO-5, both previously FDA cleared, are intended for use in 1.5 to 3 mm and 3 to 5 mm diameter vessels, respectively.
“LOBO represents the next evolution in vascular embolization. As opposed to coils, which rely on the formation of an irregular mass with relatively large voids to try to occlude vessels, LOBO utilizes a high density, uniform small pore structure to occlude vessels nearly instantly,” said Raj Pyne, MD, FSIR, interventional radiologist at Rochester Regional Health. “The ability to occlude vessels quickly and consistently with a single-device not only improves procedure efficiency, but also is critical and often life-saving in situations such as trauma and unstable bleeding. The smaller occluders, LOBO-3 and LOBO-5, have demonstrated excellent performance and I am excited to see the larger sizes in use.”
“The FDA clearance of LOBO-7 and LOBO-9 is a testament to Okami Medical’s commitment to provide patients and physicians with access to advanced technologies that address the numerous challenges in peripheral vascular occlusion,” said Bob Rosenbluth, PhD, President and CEO of Okami Medical. “The LOBO system now includes a portfolio of devices that is specifically designed and built to quickly and completely occlude a wide range of vascular targets, thus eliminating the need for multiple embolic devices and enabling more efficient interventions.”
About Okami Medical
Okami Medical, Inc. is a privately-held medical device company focused on developing innovative solutions that address key unmet clinical needs in peripheral vascular intervention. Okami is the second portfolio company of Inceptus Medical LLC, a medical device incubator. Inari Medical, Inc. (NASDAQ: NARI), Inceptus’ first portfolio company, is pioneering approaches to treat pulmonary embolism and venous clotting. Okami is backed by members of the board of directors, U.S. Venture Partners (www.usvp.com) and medical device industry veterans. For more information, please visit www.okamimedical.com.
Source:
Okami Medical, Inc.
949-446-9710
info@okamimedical.com


